Summer Research Report
My project focused on investigating how trace elements of fluorine can impact the growth of calcium carbonates in aqueous solutions. Calcium carbonates are an important biomineral found in water and are critical for many ecosystems. They play an important role in the natural world. They are the fundamental material used by marine organisms to create corals and skeletal structures, they are a key element of the carbon cycle, aid in the formation of sedimentary rocks such as chalk and limestone, they maintain soil equilibrium and can form beautiful cave systems. Fluorine is a natural element that can be frequently seen in dental health products, agrochemicals, electronics, plastics and smelting. While in small amounts fluorine is harmless, in my study our findings are indicative that fluorine can have a damaging effect on the kinetics of calcite growth and can produce changes in the crystal morphology. We discovered this through several types of experiments; we used UV-VIS spectrometry, X-ray Diffraction (XRD), scanning electron microscope (SEM), and filtration.
1. The first step in this research project was to create the solutions we would be using in the experiments.
-
Calcium Chloride (CaCl2)
-
Sodium Carbonate (Na2CO3)
-
Sodium Fluoride (NaF)
These 3 solutions were the foundations to all my experiments. By adding CaCl2 and Na2CO3 we are able to create calcium carbonates. To add dilutions of fluorine to the solution we would mix a ratio of NaF to the Na2CO3.
2. UV-VIS spectrometry allowed us to time the formation of calcium carbonates. This was the first experiment we conducted. It measures the amount of light passing through a cuvette that contains one of the dilutions. As time passes less light is able to go through as crystals form and cause tiny disruptions. While this experiment is running the machine is connected to a software that tracks the time and visibility, creating a live graph. In doing this experiment we were able to obtain quick and accurate data. The first few rounds were able to tell us that as we increased the amount of fluorine, the amount of time to form crystals was increasing.
Fig1. UV-VIS Spectrophotometer Fig 2. Real-time data being tracked
Fig 3. Dilutions of Fluorine.
Fig 4. Graph of dilutions 0-5mM of fluorine.
Fig 5. Graph of 0,1,3,5 and 7mM of fluorine demonstrating the increased length of time for each reaction to complete.
We saw that there was clear correlation between the higher the amount of fluorine and the longer length of time for the formation of calcite. We first saw this in the kinetics experiments with UV-Vis.
3. Once we had identified the elongation of the formation of the crystals we then decided to filter the solution and use the XRD to analyse the chemicals present. XRD works by sending x-rays through a sample. When one of these x-rays meets a chemical it bounces off and creates a unique spike that relates to a specific element. It allows us to identify the chemical build-up of samples.This confirmed that there was calcite forming.
The large spike labelled “104” is indicating the presence of calcite.
Fig 6. Graph from the XRD
Fig 7. XRD machine Fig 8. Samples prepared for XRD
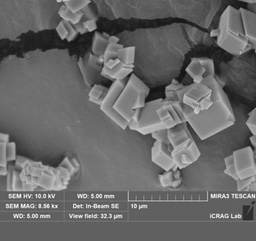
4. With this established we took the samples used for the XRD and prepared them for the SEM to examine the morphology. The preparation for the SEM requires stubs with a tiny amount of the sample to be coated in either gold or carbon coating. This coating allows the SEM to create a more accurate image. Several samples can be placed on one stub. To navigate the stub we create a detailed map to reference once the sample has been placed in the vacuum. The SEM technician helped to set-up and focus the lens and then we were able to freely explore the samples. This high-powered microscope is able to see miniscule things. It works in a manner not unlike the XRD. It shoots a beam of electrons at the sample. These electrons bounce off the object in different directions and a detailed image is created from this information.
We found that with higher concentrations of fluorine the physical appearance of the calcite would become more and more cracked and dilapidated. In particular the surface of the typically smoothe crystals had fissures and fractures.
Fig 9. Prepared stubs coated in gold
Fig 10. Typical carbonates Fig 11. Typical carbonates
Fig 12. Fig 13.
Fig 14. Fig 15.
Fig 16. Fig 17.
Fig 18.
5. The final findings of my project found that even trace elements of fluorine have effects on the formation of calcium carbonates both on a kinetic and morphological measure. Defects in the morphology of calcium carbonates can have significant impacts on the environment and systems it inhabits. In the carbon cycle calcites play a crucial role in regulating CO2 level by trapping carbon dioxide inside of itself as it forms in ocean systems (Fig 10 and 11 are typical formations of calcites). If it is unable to do so due to morphological flaws caused by fluorine (see fig 12-18) this could have a serious negative effect on the climate crisis. Similarly calcium carbonates in soil typically help to neutralise acidic soil and balance pH levels which are fundamental to soil health. Disrupted calcites are unable to execute these roles to the same standard and without them agricultural practices will become progressively less fruitful and ultimately unfeasible.
My finished project, remarkably, obtained all the data and objectives I had set out to find. In addition to this my research has opened up doors for further research with my supervisor with the possibility of co-authoring a second paper down the road. I am hopeful that this data will be published in a paper with my PI within the next 18 months and my research will bring much needed attention to the environmental impacts of fluorine on our waterways.
I received training on all the apparatus I required for my project as well as having the opportunity to participate and learn from PhD students and fellow researchers.
I achieved both personal and professional goals during this summer project. I was unsure in my abilities to participate in this research. I was overwhelmed by my lack of experience and knowledge on a topic that my PI had dedicated his professional life towards. It was incredibly daunting to start working with such esteemed scientists. However, I will say that as time passed I became more comfortable talking and discussing matters. I was confident in asking for clarification on certain terms or processes I didn't understand and not being afraid that I would expose myself as an “imposter” for not simply not knowing something. Personally I feel as though I have developed a sense of self-assurance as a result of pushing myself out of my comfort zone and committing to the experience.
In a professional aspect I have gained invaluable amounts of exposure to lab work and academic research. I was collaborating with a team of highly qualified PhD students, professional researchers, masters students and postdocs. They were all extremely warm, welcoming and encouraging of my research. I feel comfortable contacting and consulting with them on any further projects which I think is a great connection to have been able to create. There were several overlaps between our individual projects so a strong sense of comradery was formed. I was able to participate in their research as they were equally able to do so in mine. This had led to me being involved in 3 papers. This is an incredible achievement as an undergraduate.
This research project has provided me with an insight as to how I am as a researcher. My biggest take -aways that I learned about myself were
-
The importance of self-belief and self-confidence.
The difference in my confidence levels from the beginning of the summer to now are sizable. I had this notion in my head that to have belief I had to be an expert and know everything there is to know about my given topic. This is definitely a factor to being self-assured but a much larger part of it is, in my opinion, believing you have every right to be there. Nobody can magically become an expert, it takes time and experience to build up that knowledge. The people that I look up to and feel inadequate beside were once standing in my shoes. This change in mindset took time but had a significant impact on how I conduct myself as a person.
-
Problems are unavoidable
No matter how well planned out a project is or how perfectly you measure and calculate there is no avoiding hiccups along the way. I was very fortunately blessed with the kindest and most encouraging supervisor, who never let me worry for a second if something didn’t go according to plan. If a set of results did not align with what we were expecting he would simply say “we are entitled to our mistakes” and that this data is still “telling us a story”. He had such a unique spin on any issues that they never even seemed like problems or challenges at the time. This is a trait I aspire to acquire and maintain in my academic career.
-
Don’t be afraid to kill your babies
A vital element of being a good researcher is being able to objectively question and analyse your findings. Sometimes you can trap yourself into a fruitless endeavour simply because you aren’t able to call time of death on a method. I believe it is important to be able to take a step back and realise if the angle you’re coming at is working or if you are forcing it to work. I didn’t personally experience this on my project but it came up in a conversation with a colleague and I fully took it on board as it felt it was sage advice.



Please sign in
If you are a registered user on Laidlaw Scholars Network, please sign in