Laidlaw Reflections: Some secrets from the lab
The six weeks in brief:
The gene from a type of virus (crAssphage) was successfully cloned in bacteria. Then, the bacteria were stimulated to express that gene, i.e. produce the virus enzyme. The enzyme was shown to be active. Next steps could be to investigate the enzyme properties under different conditions and quantify its catalytic activity.
Some stories...
Who doesn’t like surprises?
Me. What kept surprising me, I mean in addition to the fire alarm testing every Wednesday at 2 pm, were the gels.
Figure 1. Loading my protein samples on a gel to be analysed by SDS-PAGE.
The products of my experiments were either DNAs or proteins, which I could identify by gel electrophoresis. Basically, you make the gel, load the sample and apply electric current. The results show the weight/length of the products relative to a molecular marker and a control compound. Nothing complicated, but as an undergraduate student (feeling like a radioactive element) I sometimes tended to do tiny mistakes. As a result, in the end I would have a completely different results than expected. The problem is not exactly if the results would be as expected but if they would be reliable, which could be judged by looking at the control samples. So, when they apparently were not such, my supervisor would list all the possible explanations, finishing saying – ‘or, although unlikely, you might have forgotten to add X’...
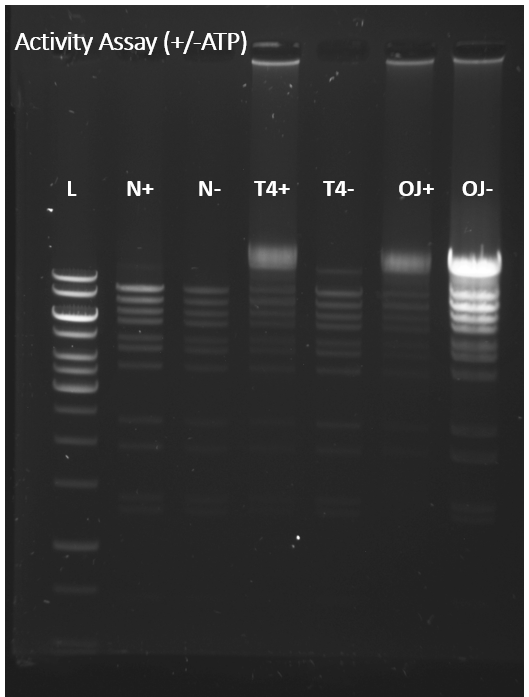
Figure 2. Agarose gel of the products after the enzyme catalysed reaction of ligating of DNA fragments (joining shorter DNA fragments together). The OJ- column was expected to be similar to T4- and the controls (N+/-), as the buffer did not contain ATP (a vital cofactor of the enzyme). However, I had made a mistake, probably, while mixing the reagents and the results looked unreliable. If these results were correct, they would imply that the enzyme is more active in the absence of the cofactor. Thus, they would be controversial to the common scientific beliefs.
.png)
Figure 3. The fourth repeat of the same experiment. This time lane OJ- looks similar to T4- and the controls (N+/-), which is consistent with the expectations and more reliable.
Summer at 4°C
It is a sunny morning in late June. I wake up, put on my pullover, then my sweatshirt, and go to the lab. I have never imagined myself wearing such warm clothes in the summer. The plan for the day is to prepare a soluble protein extract and purify the protein of interest (crAssphage DNA ligase). During classes we have studied how unstable the proteins are, but no one has ever mentioned that if you happen to work with proteins, the experiments have to be carried out in a cold room at around 4°C. The fact that I work slowly does not help much and I spend an hour there…

I wouldn’t say it was a real challenge and I definitely exaggerate the story, but it required quite a bit of concentration. On the other hand, the experiment itself was more than interesting and the cold motivated me to work more efficiently. I had no choice but ignore all the surroundings and fully immerse in performing the steps quickly and accurately.
Creating Mutants
This part of the experiment confirmed what I had already supposed – my lab supervisor Dr MacNeill had honed his patience to perfection. I had no choice but improve mine, as well.
Biologists quite often knock something out to understant its function. I had to mutate the DNA encoding the enzyme in such a way that the enzyme couldn’t perform its function anymore. Although it sounds complicated, the biotech industry has developed to an extent that you can buy a site-directed mutagenesis kit and simply follow the instructions. The same way a chef follows the recipe. The difference is that the ‘ingredients’ were in tiny volumes (a thousand of a millilitre), which I could barely see, if at all. And as everything was going smoothly because of my brilliant strategy (adding the first solution to all of the tubes, then the second, etc.), my mind would suddenly distract and… I would for example add twice the needed amount of the DNA primer, or forget if I had already added the reagent. Then, I needed to discard all the samples and repeat everything from the beginning.
Of course, I had many other opportunities to test my patience but that is how science works. My summer could not have been more exciting and I have become absolutely convinced that I have chosen the right career path for me.
I would take this opportunity to express my gratitude to the Laidlaw Foundation and Lord Laidlaw. Not only was this the most exciting summer I have ever had, but also the most valuable one in terms of developing my academic and leadership skills. Being a Laidlaw Scholar is an amazing experience, that deserve all the hard work behind it. I would like, as well, to thank to the best supervisors Dr Stuart MacNeill (lab work) and Dr Carolin Kosiol (bioinformatics). I had the huge luck working with you! Thanks for all the explanations, support and positivity!





Please sign in
If you are a registered user on Laidlaw Scholars Network, please sign in