These past few weeks of research have been the most enjoyable of my summer project thus far, and so I have decided to continue my project outside of the original six-week timeframe. I have chosen to do this primarily to conduct the amyloid-β condition (which I did not initially plan to do), and also because I have been enjoying it so much that I have taken additional images to analyse. My previous blog post focused on collating the data, but since then I have focused on analysing this data and drawing conclusions from my research. As a psychology student who loves statistics and data analysis, I was looking forward to working on my research paper's results and discussion sections and found some very interesting - and somewhat surprising - results.
Here is a summary of the conditions I explored within my project-
|
Stressor |
|
|
|
|
|
|
|
Homocysteine on SH-SY5Y cells |
Untreated |
T3 |
Hcy+T3 |
PT3 |
Hcy+PT3 |
Hcy |
|
Copper on HT-22 cells |
Untreated |
T3 |
Cu+T3 |
PT3 |
Cu+PT3 |
Cu |
|
amyloid-β (Aβ) on HT-22 cells |
Untreated |
T3 |
Aβ+T3 |
PT3 |
Aβ+PT3 |
Aβ |
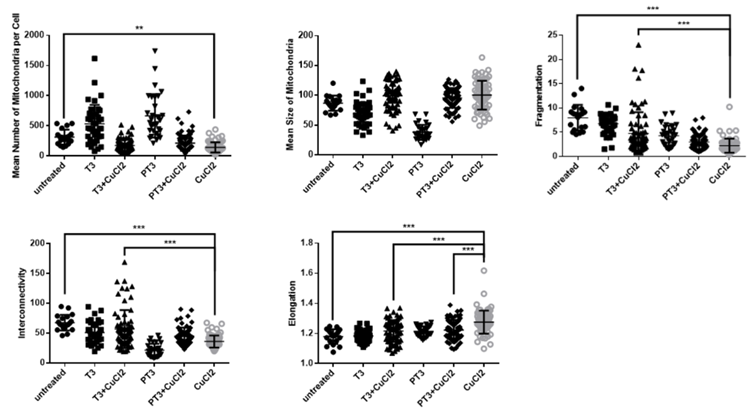
Across the three stressor variables of my project, I had over 1,100 cells to analyse; therefore, from this list of data points, there was no apparent relationship between them. Consequently, I created 30 bar graphs which presented the breakdown for the frequency of each condition for each stressor and bar graphs of the percentage of each condition relative to the untreated cells (control). This was useful as it provided a convenient way to compare my datasets and an overview of what I was working with. Then, I used GraphPad PRISM 5 software to create the scatter graphs for my research paper, and conduct the Analysis of variance (ANOVA) data examination of the mitochondrial morphology.

As SH-SY5Y and HT-22 cells are highly active, they are sensitive to alterations in mitochondrial dynamics, and this was highlighted in the graphs and statistical analysis. As expected, the stressors alter the mitochondrial morphology in all areas examined- mean number, mean size, fragmentation, interconnectivity, and elongation. Interestingly, they had different effects on these variables. For instance, all the stressors decreased the mean number of mitochondria per cell. However, the homocysteine and copper increased the fragmentation, whereas the amyloid-β decreased it. Conducting further research to explore how and why these differences occur would be interesting.

After creating the graphs and conducting the analysis, I wrote my discussion. I usually find writing lab reports to be the most enjoyable part of the writing process as it is the area where I can evaluate my work and be creative. I was surprised to see that my project produced a ‘solid’ set of results and that there was a clear pattern in my data. It is important to acknowledge that my hypothesis was correct; however, there was a finding which I did not expect - but this was a positive and constructive finding! I hypothesised that cells treated with T3 would increase mitochondrial health. When cells were treated with T3 alongside the stressor condition, there was a pattern of T3 influencing mitochondrial activity by modulating and sustaining typical mitochondrial morphology, highlighting the protective role of thyroid hormone in regulating mitochondrial dynamics. However, cells exposed to T3 for an additional 24 hours before it was treated alongside the stressor destroyed the morphology and mimicked the morphologies of cells treated with just the stressor. I was pleased and shocked to find these results, not only because it meant that there was a relationship between them, but that it potentially sets up more research and further exploration in the future.
If longer exposures to T3 have detrimental effects on the mitochondria, long-term use may not be safe. Therefore, it would be beneficial to look at this in greater detail to ascertain this, potentially looking at different concentrations and exposure times of T3. Hyperthyroidism-like problems appear to be present in the three metabolite conditions. It is known that hyperthyroidism inflicts structural and functional damage to mitochondria, eventually leading to energy depletion and cardiac dysfunction, which is significantly associated with an increased risk of AD (Maity et al., 2013; Qiu et al., 2006). My results link to this previous research, however no one has studied this particular link before and so further research is needed to fully understand the molecular links and mechanisms between the metabolites, T3, and mitochondrial dysfunction. However, the present results contribute toward understanding these risk factors on a cellular level, which can open new pathways in drug discovery. Looking at the impact of the stressors provides evidence to have a more targeted approach to the precise lifestyle modifications that can be made to reduce the probability of developing dementia and improve brain health. This is something I am looking forward to focusing on for my leadership in action project next summer.

Three Key Takeaways from my Project:
- Gained a greater insight into the scientific process, from developing a research question to working in a lab to reporting my findings
- It’s ok to fail. Throughout my Laidlaw project I realised that setbacks were a normal part of the learning process. I analysed what went wrong, and put these mistakes into my growth perspective, which helped me build my project
- Seeking continuous advice and feedback to reflect on my work is necessary for development and improvement
I have thoroughly enjoyed working on my project and I am so grateful for the support I have received during the research process. Overall, it was very insightful and has made me realise that working neurodegenerative research is the area I would like to go into in the future.
References:
Maity, S., Kar, D., De, K., Chander, V., & Bandyopadhyay, A. (2013). Hyperthyroidism causes cardiac dysfunction by mitochondrial impairment and energy depletion. Journal Of Endocrinology, 217(2), 215-228. DOI:10.1530/joe-12-0304
Qiu, C., Winblad, B., Marengoni, A., Klarin, I., Fastbom, J., & Fratiglioni, L. (2006). Heart Failure and Risk of Dementia and Alzheimer Disease. Archives Of Internal Medicine, 166(9), 1003. DOI:10.1001/archinte.166.9.1003





Please sign in
If you are a registered user on Laidlaw Scholars Network, please sign in