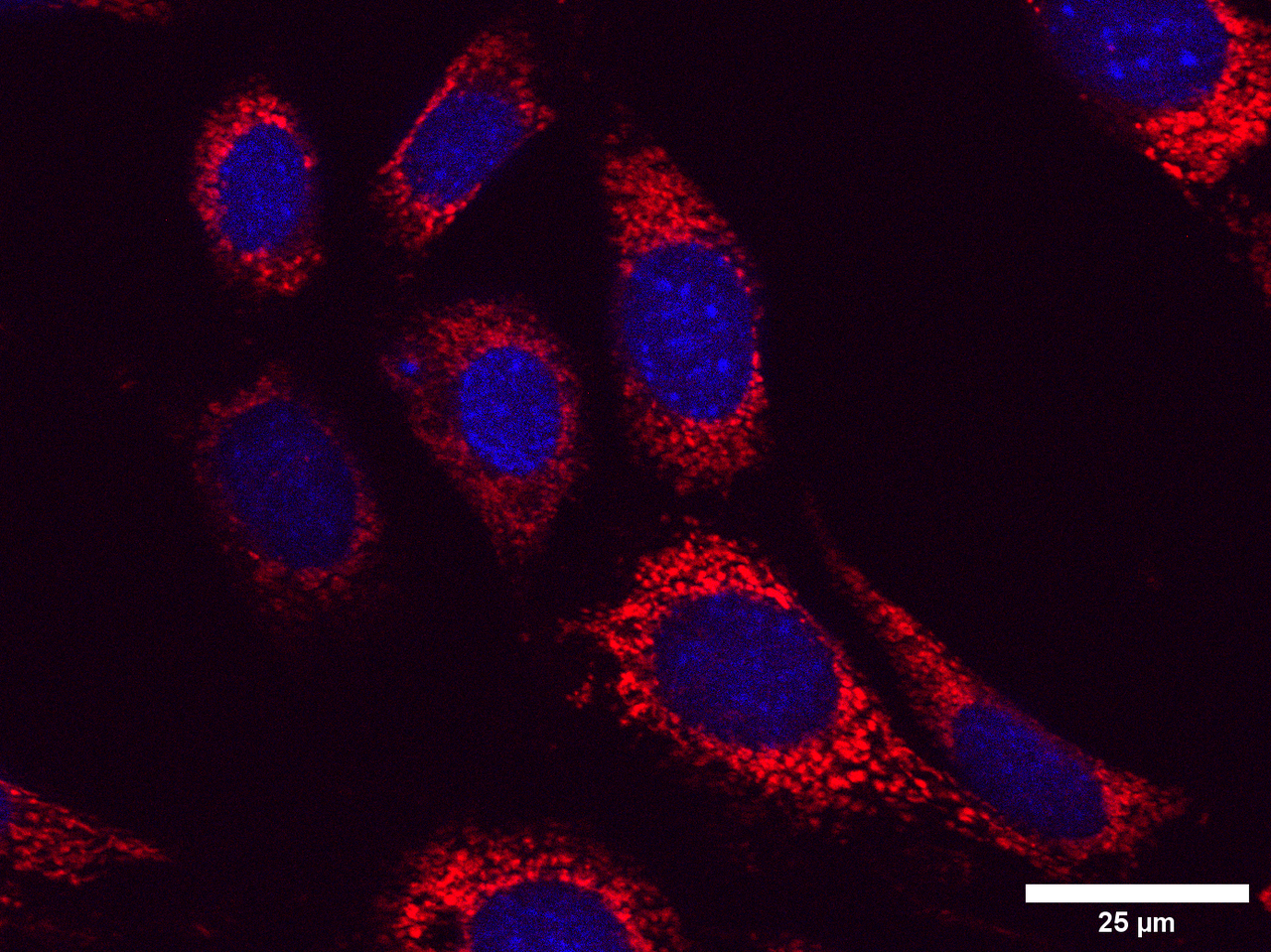
When I started on my Laidlaw summer research project, I did not expect that I would be spending hours sitting in a cold and dark room using a Zeiss microscope, taking over 250 images of cells. These images show undifferentiated SH-SY5Y neuroblastoma cells and undifferentiated HT-22 hippocampal cells treated with thyroid hormone and copper/homocysteine (kill treatments aimed to mimic features of Alzheimer's disease). During the first week of my research, I was very overwhelmed with the workload, but at the same time, I was excited to take on such a challenge. Looking back, I am pleased with the progress I have made in the past three weeks and how I overcame the initially daunting workload of the project.

The first two weeks of the project posed the biggest challenge. I struggled to use the microscope as I had not used one in over three years - resulting in several setbacks during the lab process. This taught me a great degree about being 'thrown in the deep end' - it can be one of the best ways to learn new skills, and in this instance, it helped me learn how to use a Zeiss and its compatible software. To help manage the overwhelming nature of this new experience, I spent the first week of the project learning how to use the equipment to become more familiar with it. Reflecting, this was hugely beneficial because it made me more efficient and productive when imaging the cells during my lab sessions.
Another problem I faced during the imaging process was that I struggled to find cells for some of the conditions, such as the homocysteine and copper conditions. However, as these were the kill conditions, it was expected, but this has led to significant discrepancies in the number of cells I have for each condition. Therefore, I learned to adapt my imaging for each condition to optimise the outputs.
When imaging the cells, I used DAPI staining to find the nuclear at x20 magnification, then increased the magnification to x63 to take photographs of the mitochondria. I found this difficult at first because sometimes the images were out-of-focus, or I could not see the cells at all. Eventually, with more practice, I got into a rhythm and found it easier to distinguish the cells, image them, and get high-quality MitoTracker red images. I improved on this throughout the imaging process and slowly but surely became more confident when imaging. This is evident towards the end of the imaging process because the amount of work that took me half a day at the beginning of the project I could do in an hour two weeks later.

I have now finished the imaging process and have started analysing the images I have taken using Fiji software. This allows me to break down the images and gather details on the individual cells within the image. Despite being a very time-consuming process, I am finding the statistical and structured methodology involved in this process very enjoyable. From this, I now have data on different components of the cell. I am now beginning to explore a specific part of this further by creating graphs and conducting statistical analysis, for instance, looking at the number and size of the mitochondria and their fragmentation, interconnectivity, and elongation.
I am now approximately halfway through my project. Overcoming the challenges I faced during the project so far (particularly when imaging the cells) has been an incredibly fulfilling experience. I found that completing each slide and cell type to be a gratifying experience, and I could see that my confidence, efficiency, and the quality of my work was improving. I have enjoyed reflecting on what I have learned and looking back on the work hours I have spent sitting in a microscopy room. I am pleased with the analytical and problem-solving skills I have developed so far during the project, and I am very excited to gain more skills and knowledge as my research continues.






Please sign in
If you are a registered user on Laidlaw Scholars Network, please sign in